What allows a substance to go from one phase to another?
Fundamentals of Phase Transitions
- Page ID
- 1536
Phase transition is when a substance changes from a solid, liquid, or gas land to a different state. Every chemical element and substance tin can transition from one phase to another at a specific combination of temperature and force per unit area.
Phase Changes
Each substance has 3 phases it can change into; solid, liquid, or gas(ane). Every substance is in one of these 3 phases at certain temperatures. The temperature and force per unit area at which the substance will change is very dependent on the intermolecular forces that are acting on the molecules and atoms of the substance(2). In that location can exist two phases circumstantial in a single container at the aforementioned fourth dimension. This typically happens when the substance is transitioning from one phase to another. This is chosen a two-stage state(4). In the example of water ice melting, while the ice is melting, there is both solid water and liquid water in the cup.
There are six means a substance tin can change between these iii phases; melting, freezing, evaporating, condensing, sublimination, and deposition(2). These processes are reversible and each transfers between phases differently:
- Melting: The transition from the solid to the liquid phase
- Freezing: The transition from the liquid stage to the solid phase
- Evaporating: The transition from the liquid phase to the gas phase
- Condensing:The transition from the gas phase to the liquid phase
- Sublimination: The transition from the solid phase to the gas phase
- Deposition: The transition from the gas phase to the solid stage
How Phase Transition works
There are two variables to consider when looking at stage transition, pressure (P) and temperature (T). For the gas state, The relationship between temperature and pressure level is defined by the equations below:
Ideal Gas Law:
\[ PV=nRT\]
van der Waals Equation of State:
\[ \left(P+a*\frac{n^two}{Five^2}\right)\left(V-nb\right)=nRT\]
Where V is book, R is the gas constant, and northward is the number of moles of gas.
The ideal gas law assumes that no intermolecular forces are affecting the gas in any way, while the van der Waals equation includes ii constants, a and b, that account for any intermolecular forces acting on the molecules of the gas.
Temperature
Temperature can alter the phase of a substance. One common example is putting water in a freezer to modify it into ice. In the picture above, we take a solid substance in a container. When we put information technology on a rut source, like a burner, heat is transferred to the substance increasing the kinetic energy of the molecules in the substance. The temperature increases until the substance reaches its melting point(2). As more and more heat is transferred beyond the melting bespeak, the substance begins to melt and become a liquid(3). This blazon of phase change is chosen an isobaric process because the pressure of the organization stays at a constant level.
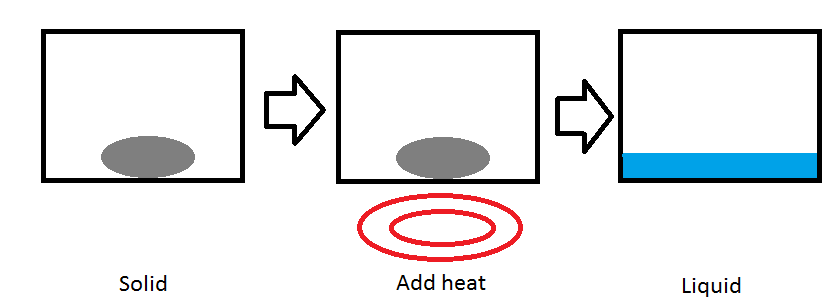
Melting signal (Tf)
Each substance has a melting point. The melting point is the temperature that a solid volition get a liquid. At unlike pressures, different temperatures are required to melt a substance. Each pure element on the periodic table has a normal melting point, the temperature that the element will go liquid when the pressure is one atmosphere(ii).
Humid Point (Tb)
Each substance also has a humid point. The boiling signal is the temperature that a liquid will evaporate into a gas. The boiling point will change based on the temperature and force per unit area. Just like the melting point, each pure element has a normal boiling signal at 1 atmosphere(2).
Pressure
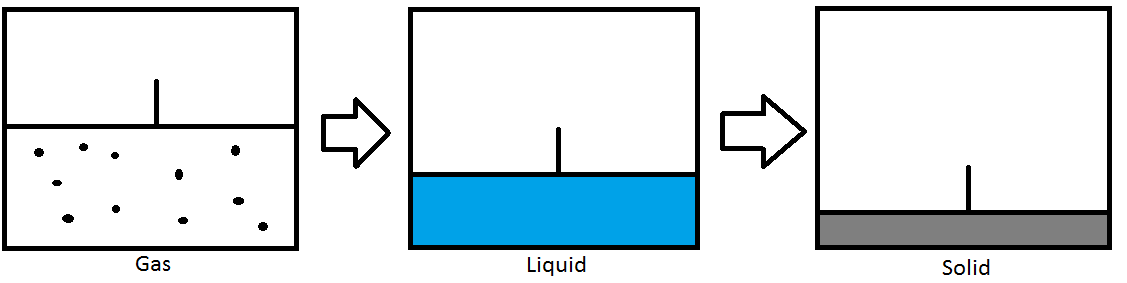
Pressure level tin can too be used to alter the phase of the substance. In the picture above, we take a container fitted with a piston that seals in a gas. Equally the piston compresses the gas, the force per unit area increases. Once the boiling point has been reached, the gas will condense into a liquid. As the piston continues to shrink the liquid, the force per unit area volition increase until the melting betoken has been reached. The liquid will and then freeze into a solid. This example is for an isothermal process where the temperature is constant and only the pressure is changing.
A Brief Explanation of a Stage Diagram
Phase transition can be represented with a phase diagram. A phase diagram is a visual representation of how a substance changes phases.
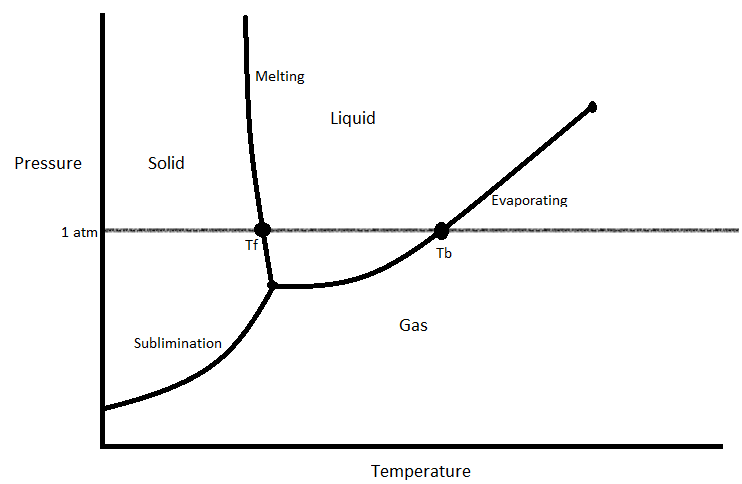
This is an case of a phase diagram. Ofttimes, when you are asked about a phase transition, yous will need to refer to a phase diagram to answer it. These diagrams usually accept the normal boiling indicate and normal melting signal marked on them, and take the pressures on the y-centrality and temperatures on the x-axis. The bottom curve marks the temperature and pressure combinations in which the substance will subliminate (1). The left left marks the temperature and pressure level combinations in which the substance will melt (one). Finally, the right line marks the conditions under which the substance will evaporate (1).
References
- Olander, Donald R. Full general Thermodynamics. Boca Raton: CRC, 2008.
- Oxtoby, David Due west., H. P. Gillis, and Alan Campion. "Phase Transition." Principles of Modern Chemistry. 6th ed. Singapore: Thomson/Brooks/Cole, 2008. 428-xxx.
- Schmidt, Philip S. Thermodynamics: an Integrated Learning Arrangement. Hoboken, NJ: Wiley, 2006.
- Sherwin, Keith. Introduction to Thermodynamics. London: Chapman & Hall, 1994.
Problems
ane. Using the phase diagram for carbon dioxide below, explain what stage carbon dioxide is normally in at standard temperature and pressure, 1 atm and 273.fifteen Grand.

Phase diagram for CO2.from Wikipedia.
2: Looking at the same diagram, nosotros see that carbon dioxide does not have a normal melting bespeak or a normal boiling signal. Explain what kind of a modify carbon dioxide makes at 1 atm and judge the temperature of this point.
Solutions
1: Earlier we can completely respond the question, we demand to convert the given information to match the units in the diagram. Kickoff nosotros catechumen 25 degrees Kelvin into Celsius: \(K=273.xv+C\) \[ 298.15-273.25C\] At present we can look at the diagram and decide its phase. At 25 degrees Celsius and i atm carbon dioxide is in the gas phase.
2: Carbon dioxide sublimes at 1 atm because it transitions from the solid phase direct to the gas phase. The temperature of sublimation at ane atm is nearly -80 degrees Celsius.
Contributors and Attributions
- Kirsten Amdahl (UC Davis)
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/States_of_Matter/Phase_Transitions/Fundamentals_of_Phase_Transitions
0 Response to "What allows a substance to go from one phase to another?"
Enregistrer un commentaire